Luna’s NanoSPRi-Based Assay Rapidly Detects Organ Injury Biomarkers with a Blood Test
by Judy M. Obliosca, Dimpal Patel, Yang Xu, Christopher Tison
Rapid and Sensitive Detection of Organ Injuries Could Save Lives
Major organ injuries such as those to the liver, lung, kidney and brain can lead to high mortality in critically ill patients. Since these injuries are internal and frequently do not present for easy identification, their misdiagnosis can be deadly. Advanced noninvasive testing with low level detection (high sensitivity) and the ability to identify the exact problem (high specificity) is therefore needed to enhance detection, particularly at early stages. Biomarkers may have predictive values before tissue injury for specific organs becomes irreversible. Identification of such biomarkers in clinical samples would improve the early detection of organ injury, help identify appropriate preventive or curative strategies, prevent organ injury from proceeding to organ failure, and improve quality of life. So far, detection of these biomarkers relies on expensive and time consuming methods such as polymerase chain reaction, mass spectrometry, bead- and absorption-based assays and enzyme-linked immunosorbent assay (ELISA). Even with their drawbacks, they still don’t have the specificity and sensitivity desired, and are limited to looking for one biomarker at a time.
The nanoSPRi Technology Fills a Critical Market Need
Luna has developed a sensing chip that monitors biomolecular interactions on its surface. Technically, it’s an in vitro diagnostic assay based on a nano-enhanced surface plasmon resonance imaging (nanoSPRi) technique. Luna’s assay immobilizes diverse capture points of antibodies and aptamers onto designated regions of the chip. When the sample of human serum is flowed across the chip, specific proteins and nucleic acids (DNA/RNA) are simultaneously trapped by their respective capture agents on the chip. This binding enables a portable, benchtop SPRi instrument equipped with a highly sensitive CCD camera to capture changes in the reflectivity on the chip in real-time. The results from the detection are displayed as a “sensorgram” (binding response on the y-axis plotted against time on the x-axis). From studying the shape of the produced sensorgram, capabilities of the technique such as binding, specificity, affinity, kinetics, active binding concentration and limit of detection were determined.
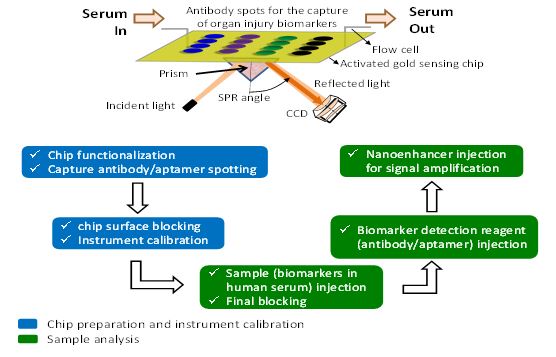
Luna’s nanoSPRi-based assay is designed for rapid, highly sensitive, and simultaneous detection of protein, DNA, and microRNA biomarkers in human serum in less than an hour of analysis. Luna’s assay consists of 3 major features: (1) The novel sensing chip surface functionalization and blocking system that can almost eliminate (>95%) non-specific binding events from human serum. (2) The array technology that enables multiplexed detection of a panel of biomarkers (both protein- and nucleic acid-based) for diverse types of organ (lung, liver, brain) injuries. (3) The nanoenhancer quantum dots (QD) that enable ultrasensitive biomarker detection at very low concentration (pg/mL). Combined, these features result in a highly sensitive, rapid, and multiplexed.
Proprietary Modifications to the Sensor Have Resulted in Significant Advances in Biomarker Analysis
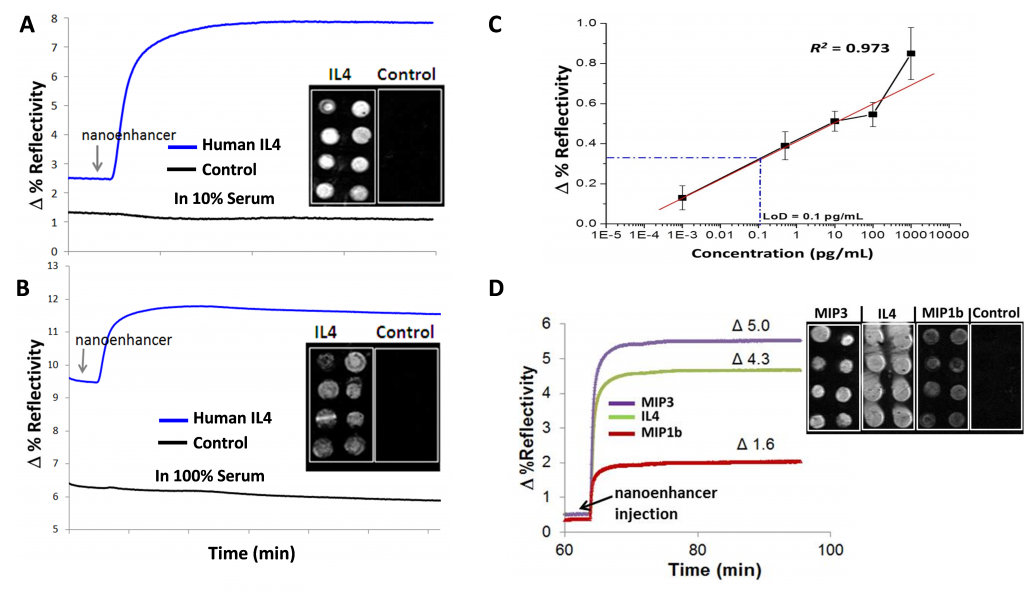
Luna has successfully developed a novel functionalization and blocking system on the sensing chip surface for use with human serum-based samples, and eliminated >95% of non-specific binding of unwanted constituents. By using the activated sensing chip, the inflammatory biomarker human interleukin 4 (IL-4) was successfully detected in less than an hour for both dilute (10%) and whole (100%) human serum samples. The assay achieved a 0.1 pg/mL IL4 limit of detection, which is 2 orders of magnitude better than that obtained using a typical ELISA. Luna also successfully achieved simultaneous detection of 6 inflammatory and acute lung injury biomarkers (IL4, MIP3, MIP1b, TNF-α, IL1β and IL8), 2 nucleic acid-based liver injury biomarkers (the DNA counterpart of miR122 and the human angiopoietin-like 3) and 1 traumatic brain injury biomarker (eotaxin CCL11) in 10% human serum with great sensitivity, selectivity and high S/N ratio. Moreover, Luna successfully integrated protein and DNA/RNA biomarker detections in serum on a single sensing chip with minimal cross-reactivity issue.
Luna’s nanoSPRi Assay is Posed to Revolutionize Advanced Biomarker Detection in Multiple Fields
Overall, Luna’s superior results demonstrate that the assay is a promising platform for accurate early diagnosis of multiple organ injuries. To date, this has been demonstrated with high sensitivity detection of biomarkers indicated in lung, liver, and kidney injuries. Further, our recently successful detection of the brain injury biomarker eotaxin CCL11 demonstrates capability for use in military and sports head trauma injury analysis. For example, CCL11 was recently shown to be predictive of chronic traumatic encephalopathy, or CTE, and has become a critical focus in brain injuries to professional athletes. This diagnostic capability being developed by Luna is a platform technology, and upon successful implementation for organ injuries, could be reconfigured for the study of a wide variety of diseases in various body fluids.
This work was supported by the US Army Medical Research Acquisition Activity (USAMRAA) under Contract No. W81XWH-14-C-0146. The views, opinions, and findings contained are those of the author(s) and should not be construed as official USAMRAA position, policy, or decision unless designated by other documentation.